Abstract
Background: Myeloproliferative neoplasms (MPN), including essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF), are at high risk of thrombotic and cardiovascular events including acute myocardial infarction (AMI). However, short-term outcomes after AMI have not been thoroughly characterized in this patient population. Our study aims to investigate differences in major adverse cardiovascular events (MACE), bleeding and thrombotic events between the different MPN phenotypes.
Methods: We conducted a retrospective cohort analysis of the National Inpatient Sample (NIS) of adult patients with a primary diagnosis of AMI from September 2015 to December 2018 with a history of MPN (N = 3,156). Patients with MPN (ET, PV, PMF) and admissions for AMI were identified using ICD-10 codes. Primary outcome was composite of inpatient MACE, a composite of arterial thrombotic event (ATE) and venous thrombosis embolisms (VTE), non-fatal cardiac arrest, and mortality. Secondary outcomes were in-hospital bleeding event requiring blood transfusion, ATE, VTE and in-hospital mortality. Outcomes between the three MPN phenotypes were compared using ET as referent group. Multivariable logistic regression models were employed. For MACE, ATE and VTE age, sex, race, hypertension, cancer, diabetes mellitus, coronary artery disease, prior PCI, prior VTE, history of renal failure, history of heart failure (HF), obesity, private insurance status, Charlson co-morbidity index (CCI), length of stay (LOS), STEMI presentation and in-hospital cardiogenic shock were used as co-variables. For mortality the same co-variables were used in addition to in-hospital ATE, VTE, and bleeding. For bleeding model age, sex, race, hypertension, liver disease, history of peptic ulcer disease, history of malignancy, history of diabetes, prior PCI, atrial fibrillation, STEMI presentation, private insurance status, smoking history, history of CKD, history of CAD, any in-hospital revascularization, prior VTE, CCI, LOS, and cardiogenic shock were used as co-variables.
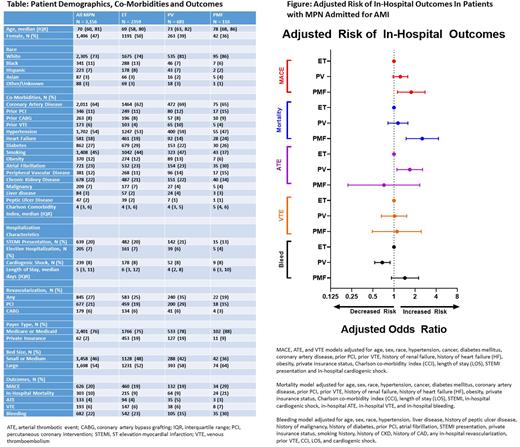
Results: There were 2359 patients with ET, 681 with PV, and 116 with PMF. Median age of ET patients was younger than PV and PMF (69 vs 73 and 78 years, respectively). Patient characteristics described in Table. MACE occurred in 626 (20%) of all MPN patients (19% of ET, 19% of PV, 29% of PMF). ATE occurred in 133 (4%) of all MPN patients (4% ET, 5% PV, 3% PMF), VTE in 193 (6%) of all patients (ET and PV 6%, PMF 7%), and in-hospital mortality occurred in 303 (10%) of all patients (9% ET and PV, 21% PMF). Bleeding occurred in 682 (22%) of all MPN patients (23% ET, 15% PV, 30% PMF). After multivariable logistic regression, patients with PV had similar risk of MACE (OR 1.23, 95% CI 0.97-1.57), mortality (OR 1.14, 95% CI 0.82-1.58), and VTE (OR 1.02, 95% CI 0.66-1.52) compared with ET. However, patients with PV had higher risk of ATE (OR 1.68, 95% CI 1.10-2.56) and lower risk of bleeding (OR 0.68, 95% CI 0.53-0.88) compared with ET. Patients with PMF had higher risks of MACE (OR 1.76, 95% CI 1.12-2.77) and mortality (OR 2.53, 95% CI 1.49-4.30) but not ATE (OR 0.72, 95% CI 0.22-2.33), VTE (OR 1.10, 95% CI 0.49-2.45), and bleeding (OR 1.43, 95% CI 0.92-2.24) compared with ET patients, Figure.
Conclusions: Patients with MPN admitted with AMI have high incidence of MACE and bleeding. Patients with ET and PV have similar rates of MACE while PMF have higher risk of MACE. Patients with PV are associated with more ATE and less bleeding risk compared with ET. More investigation is needed in order to identify risk factors for adverse cardiovascular outcomes in patients with MPNs and improved post-AMI outcomes.
Disclosures
Hobbs:Pfizer: Other: Advisor or review panel participant; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Pharmaxis: Other: Advisor or review panel participant; Keros: Other: Advisor or review panel participant; Abbvie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Constellation: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; PI, Research Funding; Bayer: Research Funding; Bristol Myers Squibb Co./Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Incyte: Other: Advisor or review panel participant; PI, Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.